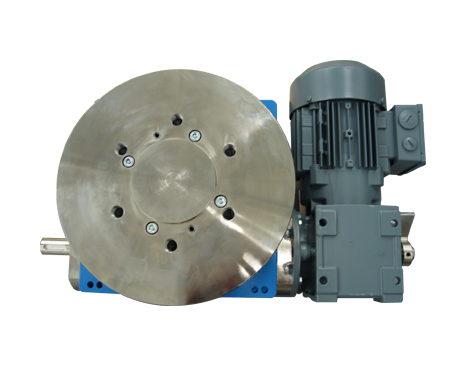
A global manufacturer of pharmaceutical dosing machines approached Motion Index Drives to resolve an issue that its North American customers were experiencing with cam indexing devices that were installed in their machines. The units that were utilized in the standard dosing machines manufactured in Europe had a sealing issue. The dosing machines are utilized to fill capsules with powder or liquids in the pharmaceutical industry from health supplement to prescription drug manufacturing. The original equipment, using another manufacturer’s rotary cam indexer, was failing do to powder and liquid breaching through the standard seals and damaging the internal mechanical components. The eight-station indexer had to be manufactured to continuously run and synchronize other apparatuses on the dosing machine and most importantly be sealed to prevent any powder or liquid from entering the internal components of the rotary indexer. The other important feature was manufacturing a unit that would fit and function (have same foot print) into the current OEM’s dosing machine without the customer having to alter anything.
SOLUTION
Motion Index Drives 8-station fixed RT160-8-90 custom engineered unit was ideal fix for the customer’s problems. We took a standard RT160 rotary cam index unit and first equipped it with a custom cam profile to match the synchronization that was required to operate the dosing machines correctly and make it function the same. Secondly, we addressed the sealing issues by manufacturing the standard RT160 unit with special double seals and a labyrinth style seal on the top rotating dial. The labyrinth seal on the top dial was engineered to eliminate the biggest problem that was experienced on the competitors units, which had the largest percentage of the powder and liquid breach occurring on the top dial do to very poor sealing. We took to engineering one step further by nickel plating the top dial and input shafts to eliminate corrosion on the units.